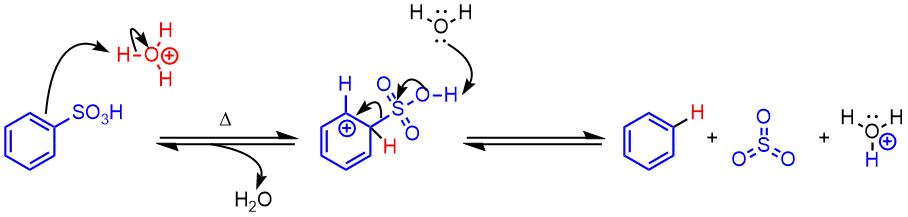
Aromatic desulfonation
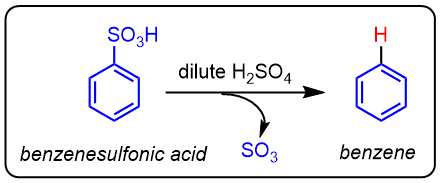
Treatment of benzenesulfonic acids with dilute acids leads to desulfonation. The sulfonation of benzene is a reversible reaction. Thus, we can remove a sulfonic acid group by heating the compound with an acid. Sometimes the reaction can be carried out with steam (frequently it is sufficient for the desulfonation of the compound).
Mechanism
- Attack by an electrophilic ion (hydronium) protonates the aromatic ring and generates a resonance-stabilized carbocation intermediate.
- Desulfonation: A base (water) deprotonates the sulfonic acid and sulfur trioxide leaves, regenerating the aromatic ring.